Co Oxidation Number
Let x be the oxidation number of Ni in NiCO 4. This sharing may include losing or gaining electrons.
The oxidation number of N 3-is -3.

. The oxidation state of OxygenO -2. For example the oxidation number of Na is 1. The sum of oxidation numbers in a neutral compound is 0.
In Mg 3 N 2 the oxidation number of Nitrogen is 3. Oxidation number of CoCl 2 NH 3 4 Oxidation number of Co 2Cl 40 1. This applies to monatomic ions as well as complex ions.
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to a more electronegative element. The alkali metals group I always have an oxidation number of 1. The usual oxidation number of hydrogen is 1.
Calculating the oxidation number of CarbonC in Carbon monoxide CO. C The oxidation number can be defined as the number of electrons available in any compound that is shared with another compound to form a chemical bond. Oxygen almost always has an oxidation number of -2 except in.
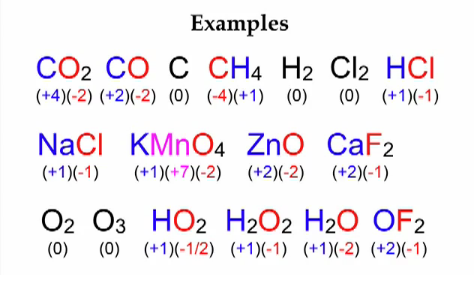
The oxidation number of fluorine is always 1. The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion. Since the CO 2 molecule is neutral the carbon atom must exhibit an oxidation state of 4 the sum of all the oxidation numbers in a neutral molecule is zero.
We may find oxidation number in a way like oxidation no of CO can be calculated as CO Oxidation no of known ekement oxidation no of unknown 0 Consider the oxidation of carbon is x than x -2 0 Then x 2 Therefor oxidation no of CO is 2. Numbers are very useful. The oxidation number of a Group 2 element in a compound is 2.
H2O2 where it is -1. In the ion CO 3 2- C has an oxidation number of 4 and the three O each have an oxidation number of -2. To calculate oxidation numbers of elements in the chemical compound enter its formula and click Calculate for example.
However most metals are capable of multiple oxidation states. Thus the oxidation number of Ni in NiCO 4 is 0. The alkaline earth metals group II are always assigned an oxidation number of 2.
The oxidation number of a free element is always 0. A 1 b 3 c -1 d -3 Ans. Ca2 HF2- Fe4 Fe CN63 NH4NO3 so42- ch3cooh cuso45h2o.
The oxidation number of the monatomic ion F-is -1. Oxidation number also referred to as oxidation state is the number that is allocated to elements in a chemical combination. Typically this relates to the number of electrons that must be gained negative oxidation number or lost positive oxidation number for the atoms valence electron shell to be filled or half-filled.
The oxidation number refers to the electrical charge of an atom. When it comes to redox reactions there are a few reasonably effective numbers such as oxidation numbers. Since the overall charge on the complex is 0 The sum of oxidation states of all elements in it should be equal to 0.
Hence x 0. Answer 1 of 7. The oxidation number of cobalt in KCoCO 4 is.
For example to calculate the oxidation number of chlorine in HClO₄ we would take the average of VII and VII which would give. Chlorine bromine and iodine usually have an oxidation number of 1 unless they. The oxidation number of atoms occurring more than once in a molecule and having identical bonding can be calculated by taking the average of all the oxidation numbers of that atom in the molecule.
The oxidation number is synonymous with. In all Metal carbonyls the oxidation number of metals is zero. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds.
The oxidation number of a monatomic ion equals the charge of the ion. The oxidation number of any atom in its elemental form is 0. The sum of the oxidation numbers in an ion equals the charge on the ion.
The oxidation number for Co in CoS is 2 a divalent cobalt cation since the only anion formed from a single sulfur atom has a charge of -2. Therefore x 4 0 0. The atoms in He and N 2 for example have oxidation numbers of 0.
In NiCO 4 the oxidation number of Ni is zero. In simple words the oxidation number is the number assigned to the components in a chemical combination. Oxidation states x 2-1 40 1.
C N P and S have oxidation numbers of 433 and 2 in all carbides nitrides phosphides and sulphides respectively. Learn how to calculate or find the oxidation number of elements along with examples. 4 3-2 -2 which is the charge on the ion.
Therefore the oxidation state of oxygen was found to be -2 and the oxidation number. To find the correct oxidations number for CO Carbon monoxide and each element in the molecule we use a few rules and some simple mathFirst since the CO. To find the correct oxidation number for CoCl2 Cobalt II chloride and each element in the compound we use a few rules and some simple mathFirst since.
The Oxidation Number of Cobalt inCoCO4 is _____.

The Oxidation State Of C In Co Is X The Oxidation State Of C In Cocl 2 Is Y Then Y Youtube

Oxidation Number Of Carbon In Carbon Monoxide Oxidation Number For Co Oxidation State Of Co Youtube

In Ni Co 4 The Oxidation State Of Ni Is Youtube

How To Find The Oxidation Number For C In Co Carbon Monoxide Youtube

Oxidation Number Of Carbon In Carbon Monoxide Oxidation Number For Co Oxidation State Of Co Youtube

How To Find The Oxidation Number For C In Co Carbon Monoxide Youtube
0 Response to "Co Oxidation Number"
Post a Comment